Niels Jöns, Wolf-Achim Kahl,Wolfgang Bach
Lithos (2016) 268-271, 274-284.
https://doi.org/10.1016/j.lithos.2016.11.014
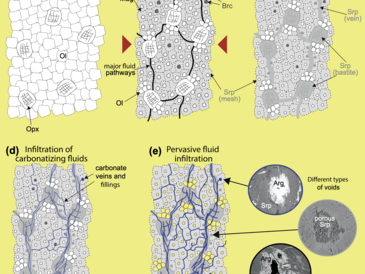
In a drillcore sample of serpentinized harzburgite from the uppermost oceanic crust (Mid-Atlantic Ridge, ODP Leg 209, Site 1270), we demonstrate using high-resolution 3D-microtomography that micron-sized open cavities are present. The development of porosity is interpreted to result from dissolution of brucite and/or olivine. Petrographic observations indicate that voids are integrated in a network of carbonate veins, the formation of which is linked to changing alkalinity in conjunction with dissolution reactions. Partial carbonate filling of pore spaces indicates that under static conditions low-temperature carbonation leads to clogging of fluid pathways and thus to a reduction in permeability. Electron microprobe analyses show that the inner walls of open voids are lined with Fe-rich precipitates. We propose that the iron in those phases was released by brucite or olivine dissolution and was subsequently oxidized and precipitated as ferric hydroxide. Thermodynamic computations show that this process may be a potential source of catabolic energy for microorganisms inhabiting serpentinites. The proposed carbonation mechanism implies that carbonate precipitation may start soon after exposure of the abyssal peridotites, when dissolution of brucite and weathering of olivine begin, and continue until the phases become inaccessible to seawater. Predicting carbonation rates of abyssal peridotites will hence require understanding of permeability reactions.

![[Translate to English:] Zur Startseite des KKBS](/fileadmin/user_upload/fachbereiche/fb3/kksb/logo/KKSBSchriftMitLogoSchwarz_90.png)