Hendrik Naatz, Sijie Lin, Ruibin Li, Wen Jiang, Zhaoxia Ji, Chong Hyun Chang, Jan Köser, Jorg Thöming, Tian Xia, Andre E. Nel, Lutz Mädler and Suman Pokhrel.
ACS Nano (2017) 11, 501–515
http://dx.doi.org/10.1021/acsnano.6b06495
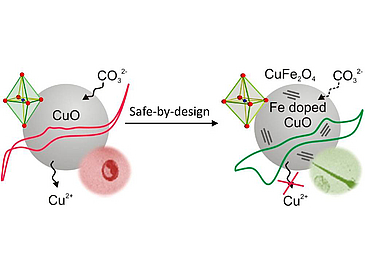
The safe implementation of nanotechnology requires nanomaterial hazard assessment in accordance with the material physicochemical properties that trigger the injury response at the nano/bio interface. Since CuO nanoparticles (NPs) are widely used industrially and their dissolution properties play a major role in hazard potential, we hypothesized that tighter bonding of Cu to Fe by particle doping could constitute a safer-by-design approach through decreased dissolution. Accordingly, we designed a combinatorial library in which CuO was doped with 1–10% Fe in a flame spray pyrolysis reactor. The morphology and structural properties were determined by XRD, BET, Raman spectroscopy, HRTEM, EFTEM, and EELS, which demonstrated a significant reduction in the apical Cu–O bond length while simultaneously increasing the planar bond length (Jahn–Teller distortion). Hazard screening was performed in tissue culture cell lines and zebrafish embryos to discern the change in the hazardous effects of doped vs nondoped particles. This demonstrated that with increased levels of doping there was a progressive decrease in cytotoxicity in BEAS-2B and THP-1 cells, as well as an incremental decrease in the rate of hatching interference in zebrafish embryos. The dissolution profiles were determined and the surface reactions taking place in Holtfreter’s solution were validated using cyclic voltammetry measurements to demonstrate that the Cu+/Cu2+ and Fe2+/Fe3+ redox species play a major role in the dissolution process of pure and Fe-doped CuO. Altogether, a safe-by-design strategy was implemented for the toxic CuO particles via Fe doping and has been demonstrated for their safe use in the environment.

![[Translate to English:] Zur Startseite des KKBS](/fileadmin/user_upload/fachbereiche/fb3/kksb/logo/KKSBSchriftMitLogoSchwarz_90.png)