Felix Zeller, Chieh-Min Hsieh, Tim Neudecker, Wilke Dononelli
Theoretical and Computational Chemistry (2023)
doi: 10.26434/chemrxiv-2023-nr314
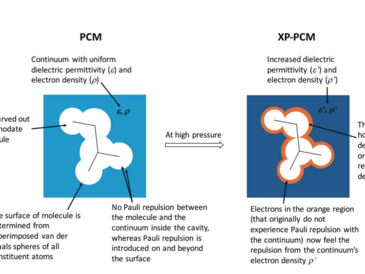
The field of liquid-phase and solid-state high-pressure chemistry has exploded since the advent of the diamond anvil cell, an experimental technique that allows the application of pressures up to several hundred gigapascal. To complement high-pressure experiments, a large number of computational tools have been developed. These techniques enable the simulation of chemical systems, their sizes ranging from single atoms to infinitely large crystals, under high pressure and the calculation of the resulting structural, electronic and spectroscopic changes. At the most fundamental level, computational methods using carefully tailored wall potentials allow the analytical calculation of energies and electronic properties of compressed atoms. Molecules and molecular clusters can be compressed either via mechanochemical approaches or via more sophisticated computational protocols using implicit or explicit solvation approaches, typically in combination with Density Functional Theory, thus allowing the simulation of pressure-induced chemical reactions. Crystals and other periodic systems can be routinely simulated under pressure as well, both in a static and in a dynamic manner, to predict the changes of crystallographic data under pressure and high-pressure crystal structure transitions. In this review, the theoretical foundations of the available computational tools for simulating high-pressure chemistry are introduced and example applications demonstrating the strengths and weaknesses of each approach are discussed.
© 2024, The Authors, CC BY 4.0