Ekaterina A. Shirokova, Alexey G. Razuvaev, Alexey V. Mayorov, Bálint Aradi, Thomas Frauenheim & Stanislav K. Ignatov
Journal of Cluster Science 34, 2029–2046 (2023)
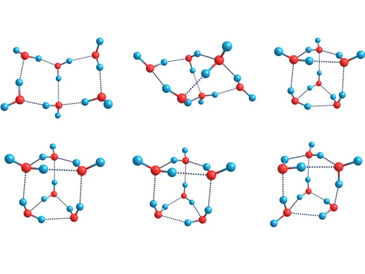
In order to estimate the effect of hydrogen bond network isomerism on the thermodynamic functions and concentrations of water clusters in the gas phase, the structural and thermodynamic parameters of all 133 possible isomeric structures of water hexamer (HO) in the initial conformations of the book, cage and prism types have been studied using the DFT (B3LYP/6-311++G(2d,2p)), DFT-D3 (B3LYP/6-311++G(2d,2p)), G4, W1BD, DFTB, and MB-pol calculations. It was found that accounting of the orientational isomerism leads to the values of water cluster gas-phase concentrations different by 1 or 3 orders of magnitude (depending on the method of energy calculation and averaging) from the results obtained when only single energetically favorable structure is considered. The absolute Boltzmann-averaged estimates of the hexamer binding enthalpy per monomer are of 5.50 (G4 results) and 5.77 (DFT results) kcal/mol. The total concentration of HO including all isomers in the saturated water vapor at standard conditions are estimated as (G4) and (DFT) molecules/cm with W1BD indirect estimates lying between these values.

![[Translate to English:]](/fileadmin/user_upload/fachbereiche/fb1/lmcqm/logos/lmcqm-logo-3-padded-up-20.png)