Ion and electron conducting hetero-aggregates for electrochemical applications
Project Leader :
Prof. Dr. Jürgen Janek
Justus Liebig University Giessen
Prof. Dr.-Ing.Arno Kwade
Technical University of Braunschweig
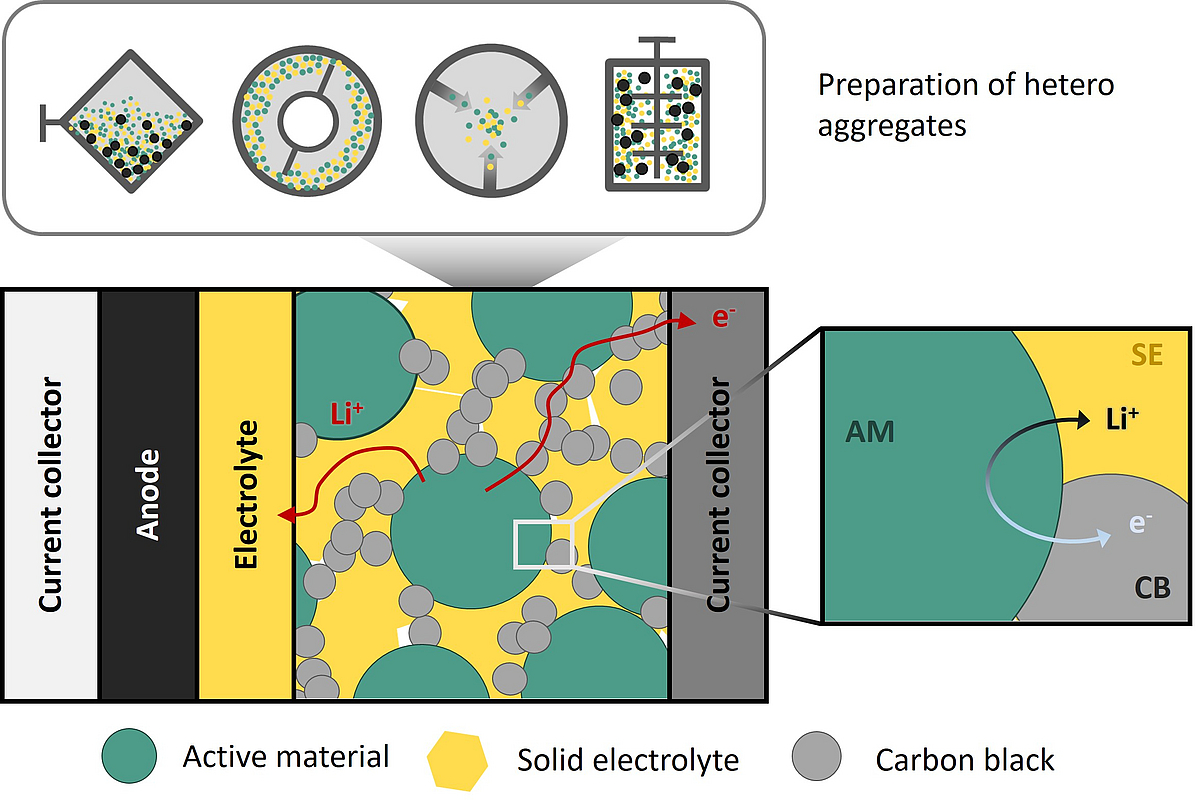
A new generation of lithium batteries, known as all-solid-state batteries (ASSB), in which liquid electrolytes are replaced by solid electrolytes (SE), is currently under intensive research as they potentially offer higher energy density and safety. A crucial component within a solid-state battery is the cathode, which is often realized as a composite consisting of active material, solid electrolyte, conductive additive, and usually a binder. The properties of solid-state batteries, particularly electron and ion transport as well as chemo-mechanical degradation, are strongly dependent on the microstructure of these composite cathodes, which is critically determined by the production process. An important step in this process is the homogeneous distribution and electronic as well as ionic contact of the active material, which can be achieved through intensive mixing processes. These processes break down larger agglomerates, leading to the subsequent formation of heteroaggregates and a uniform mixture. However, the exact influence of the submicron structure on cell performance is not well understood. Furthermore, the processes for manufacturing these solid-state cathodes and their impact on the microstructure have been scarcely studied.
In light of minimizing CO2 emissions and production costs, it is intended to manufacture the cathodes for upcoming solid-state batteries using dry processing methods. For this, an efficient and well-understood process for mixing the various solid components and thereby structuring the resulting heteroaggregates must be developed. This will be investigated in this project through various mixing processes with different stress types, intensities, and frequencies, both experimentally and through simulations.
In the first phase of the project, it was found that high-intensity stresses during mixing are necessary to avoid large agglomerates of the solid electrolyte and ensure sufficient ionic connection. Building on these findings, further mixing techniques and new material compositions are being investigated to gain a more comprehensive understanding of the mixing process. A central element of this project is the direct linkage of simulations and experiments: The stress conditions during the processing of the composites are determined, among other methods, through DEM simulations. At the same time, the composites are experimentally produced and investigated both microstructurally and electrochemically. Our hypothesis is that by knowing the stress history, the structure of the heteroaggregates and, based on this, their electrochemical performance properties can be predicted. Various mixing processes will be compared based on their stress modes. Through simulations and experiments, the necessary process parameters will be determined to make laboratory mixing methods comparable with large-scale or scalable processes. A key goal is the development of standardized mixing processes that will enable better comparability between different studies. Additionally, new material combinations will be investigated to develop more cost-effective and higher-performance batteries. The transition to less expensive electrolytes and the investigation of high-performance materials such as LiNixCoyMn1-x-yO2 (NCM) with conductive sulfide electrolytes aim to improve the performance and safety of the batteries.