Marvin Janssen; Thomas Frederichs; Marian Olaru; Enno Lork; Emanuel Hupf; Jens Beckmann
Science 385 (2024): 318-321
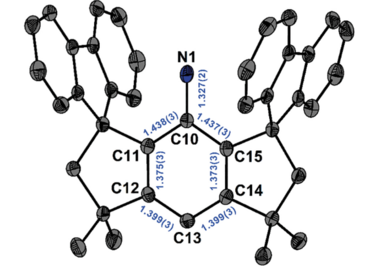
Nitrenes are a highly reactive, yet fundamental, compound class. They possess a monovalent nitrogen atom and usually a short life span, typically in the nanosecond range. Here, we report on the synthesis of a stable nitrene by photolysis of the arylazide M S FluindN 3 ( 1 ), which gave rise to the quantitative formation of the arylnitrene M S FluindN ( 2 ) (M S Fluind is dispiro[fluorene-9 ,3′-(1′,1′,7′,7′-tetramethyl-s-hydrindacen-4′-yl)-5′,9′′-fluorene]) that remains unchanged for at least 3 days when stored under argon atmosphere at room temperature. The extraordinary life span permitted the full characterization of 2 by single-crystal x-ray crystallography, electron paramagnetic resonance spectroscopy, and superconducting quantum interference device magnetometry, which supports a triplet ground state. Theoretical simulations suggest that in addition to the kinetic stabilization conferred by the bulky M S Fluind aryl substituent, electron delocalization across the central aromatic ring contributes to the electron stabilization of 2 .
© 2024, The Authors and American Association for the Advancement of Science