R. Y. Medina, I.-M., Rohdenburg, M., Mostaghimi, F., Grabowsky, S., Swiderek, P., Beckmann, J., Hoffmann, J., Dorcet, V., Hissler, M., Staubitz, A.
Inorganic Chemistry (2018), 57 (20), pp 12562–12575.
http://dx.doi.org/10.1021/acs.inorgchem.8b01649
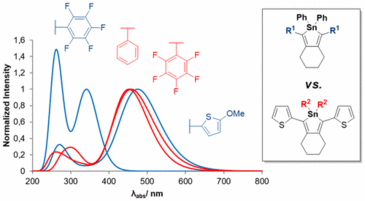
Stannoles are organometallic rings in which the heteroatom is involved in a form of conjugation that is called σ*−π* conjugation. Only very little is known about how the substituents on the Sn atom or substituents on the stannole ring determine the optoelectronic properties of these heterocycles. In this work, this question has been studied experimentally and theoretically. Calculations of optimized equilibrium geometries, energy gaps between the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs), and of the absorption spectra of a wide range of compounds were performed. The computational data showed that the substituents on the Sn atom influence the optoelectronic properties to a lower extent than the substituents in the 2 and 5 positions of the ring. These substituents in the 2 and 5 positions of the stannole ring can also have a strong influence on the overall planarity of the structure, in which mesomeric effects can play a substantial role only if the structure is planar. Thus, only structures with a planar backbone are of interest in the context of tuning the optoelectronic properties. These were selected for the experimental studies. On the basis of this information, a series of six novel stannoles was synthesized by the formation of a zirconium intermediate and subsequent transmetalation to obtain the tin compound. The calculated electronic HOMO–LUMO energy gaps varied between 2.94 and 2.68 eV. The measured absorption maxima were located between 415 and 448 nm compared to theoretically calculated values ranging from 447 nm (2.77 eV) to 482 nm (2.57 eV). In addition to these optical measurements, cyclic voltammetry data could be obtained, which show two reversible oxidation processes for three of the six stannoles. With this study, it could be demonstrated how the judicious choice of the substituents can lead to large and predictable bathochromic shifts in the absorption spectra.