Marius Wolpmann, Martin Etter, Andrea Kirsch, Filippo Balzaretti, Wilke Dononelli, Lars Robben, Thorsten M. Gesing
Zeitschrift für Kristallographie - Crystalline Materials 238 (2023): 27 - 38
https://doi.org/10.1515/zkri-2022-0037
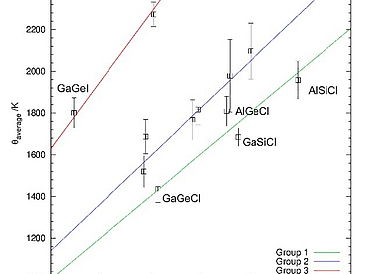
Sodalites of the general type |Na8X2|[T1T2O4]6 with X = Cl−, Br−, I− have been synthesized for Al–Si, Ga–Si, Al–Ge and Ga–Ge as T1–T2 frameworks. The structures were examined using in-house and synchrotron X-ray diffraction, Raman spectroscopy, force-field structure optimizations and DFT based ab-initio molecular dynamics (MD) computations. Calculated phonon density of states (PDOS) of the 12 compounds show only minor differences within a framework composition with a lowering of certain phonon energies with increasing anion size. Earlier published Debye and Einstein temperatures obtained with a Debye-Einstein-anharmonicity (DEA) model approach are confirmed using the determined low-temperature lattice parameters (18 K–293 K) and show no correlation with the respective PDOS. Small-box refinements against radial pair distribution functions (PDF) allowed the determination of anisotropic displacement ellipsoids (ADP) for Na+ and O2−, indicating a strong dependency of the ADP of Na+ on the chemical composition. Significantly lower thermal displacements from MD calculations suggested an influence of structural displacements. For compounds with an aspherical ADP for sodium, structural models could be refined in which the sodium is located on two 8e or one 24i site (both partially occupied), and also temperature-dependent (100 K–300 K) for the compounds with Ga–Ge framework. 3D-plots of the bond-valence sums of Na+ further validate the structural differences. These results imply that the local structure of halide-sodalites in many cases is not best described by the known average structure and may even not be cubic.