Metal Oxide Modeling
Modeling Electrochemical Oxide Film Growth
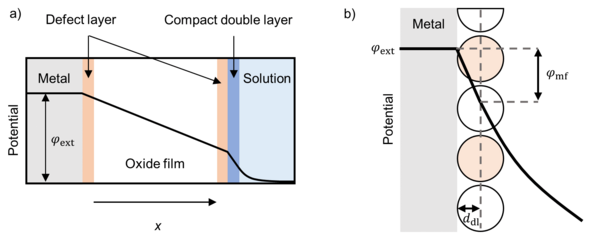
This research project is on modeling electrochemical oxide film growth at metal electrodes. Among others, oxide films play an important role in terms of corrosion and in the semiconductor industries. Modeling of oxide films can give deeper insider and understanding of the processes at the interfaces between metal and oxide film and oxide film and surrounding. Thus, corrosion protection can be enhanced and optimized.
Literature
Ingmar Bösing, Georg Marquardt, and Jorg Thöming. "Effect of heat treatment of martensitic stainless steel on passive layer growth kinetics studied by electrochemical impedance spectroscopy in conjunction with the point defect model." Corrosion and materials degradation 1.1 (2020): 77-91.
Ingmar Bösing. "Modeling electrochemical oxide film growth—passive and transpassive behavior of iron electrodes in halide-free solution." npj Materials Degradation 7.1 (2023): 53.